Just like stated up above, Bohr's planetary atomic model suggests that the atom has unchangeable energy levels. Surrounding the nucleus, there are three orbits, that each contain electrons that have a specific amount of energy. These orbits are known as energy levels, a concept that Bohr based his whole atom off of. However, one of the problems with Bohr's model was that he lacked a definite explanation as to why exactly the energy levels existed. Even with lacking that knowledge, Bohr was still able to conclude that electrons in the electron clouds each belonged to one of the different energy levels.
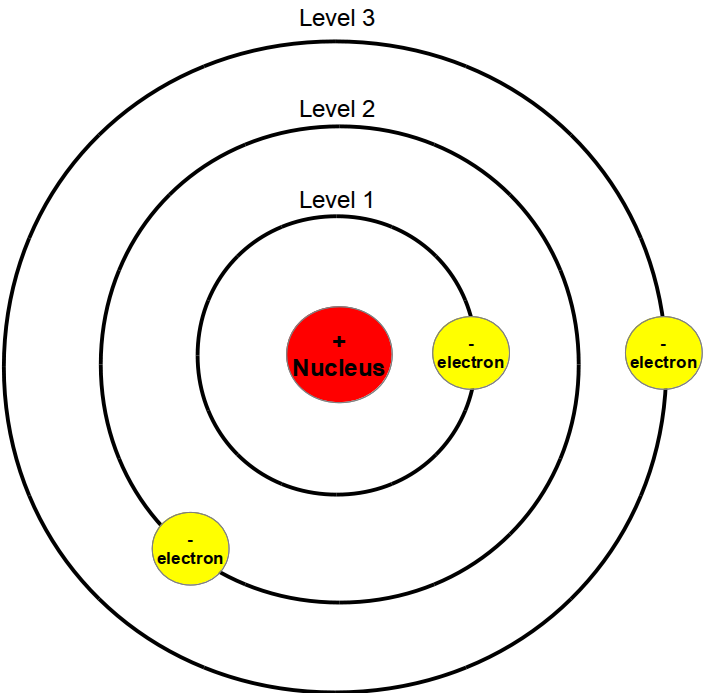 |
The Atom of the Hour: Bohr's Planetary Atomic Model!
(not so) FUN FACT: Bohr drew inspiration by comparing the nucleus to the Sun |


No comments:
Post a Comment