Since I gave my loyal readers a recipe for Bohr's model, I figured it would only be fair to include a recipe for a gourmet red velvet cake.
To begin, start with the finest quality box mix of cake that your local grocer has to offer. (I recommend buying whichever one is on sale, considering they are all practically the same mix inside a different box with a different brand name.)
Mix with oil, eggs, and water, then bake. (Hopefully that's not too hard.)
To make the frosting (from scratch!!), you'll need the following ingredients:
8 ounces of cream cheese
1 tsp. of vanilla extract
2-3 cups of powdered sugar
1 stick of butter (as if the other 3 ingredients were not fattening enough)
In case grabbing four simple ingredients is too complicated, I've attached pictures.
In case this was hard to figure out, you mix all four ingredients together (in a mixer, of all things - shocker) and then spread the frosting on the cakes (which I baked into two 8-inch circular cakes).
AND WHAT DO YOU GET? THE WORLD'S LARGEST WHOOPIE PIE!
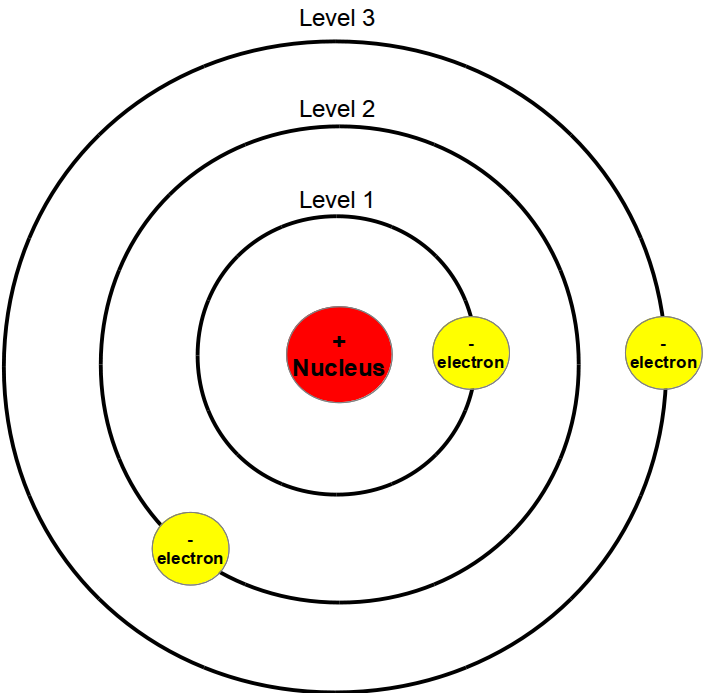
I lied, you actually get a delicious cake version of Bohr's model!
(The red center is the nucleus and the three poorly-piped circles surrounding it are the energy levels.)